The Problem
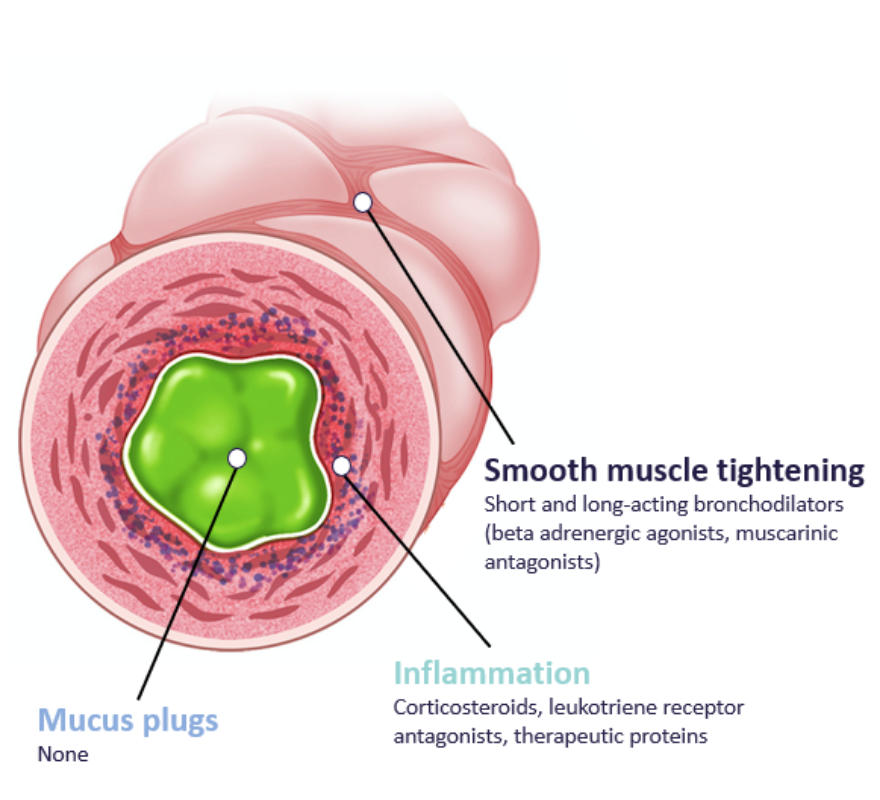
In many airway diseases characterized by inflammation and oxidative stress, mucus becomes abnormally thick and accumulates in the airways, obstructing the airflow in the lungs of patients.
This mucus thickening is caused by cross-linking between large mucin polymers in the gel and is common to many airway diseases.
The Aer Solution
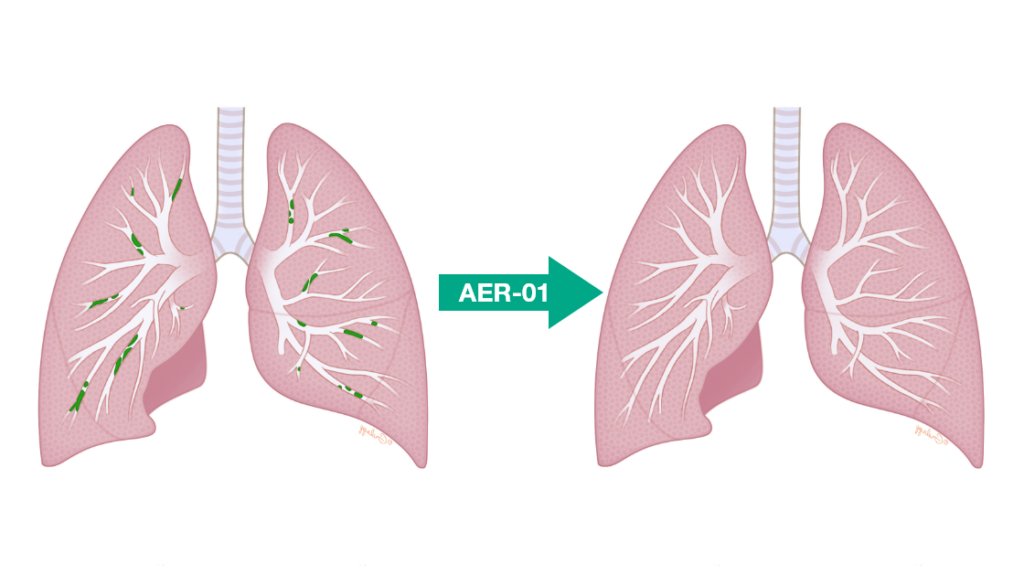
Our best-in-class, Phase I ready development candidate AER-01 has the potential to provide relief for patients suffering from respiratory conditions such as COPD, asthma and cystic fibrosis with mucus plugging.
AER-01 is a thiol-modified sugar that is delivered by nebulization or via a dry powder inhaler. AER-01 acts as a mucolytic agent by targeting and cleaving excessive disulfide cross-links between mucin polymers to liquify the plugs. Special considerations for safety, potency, and formulation for oral inhalation were made during design and development of AER-01.
The Precision Approach
New research findings show that mucus plugs are present in many, but not all, patients with COPD and asthma. This research also shows that patients with high mucus plug burden suffer from lower lung function than patients with low mucus plug burden.
Aer Therapeutics will integrate these research findings into its clinical development programs by (1) selecting appropriate patients (those with high mucus burden) and (2) monitoring the effects of AER-01 using new mucus burden measures derived from non-invasive CT imaging.
These data provide rationale for targeting patients with mucus-high /emphysema-low COPD in clinical trials of mucoactive treatments.
Dunican and Fahy, AJRCCM 2021
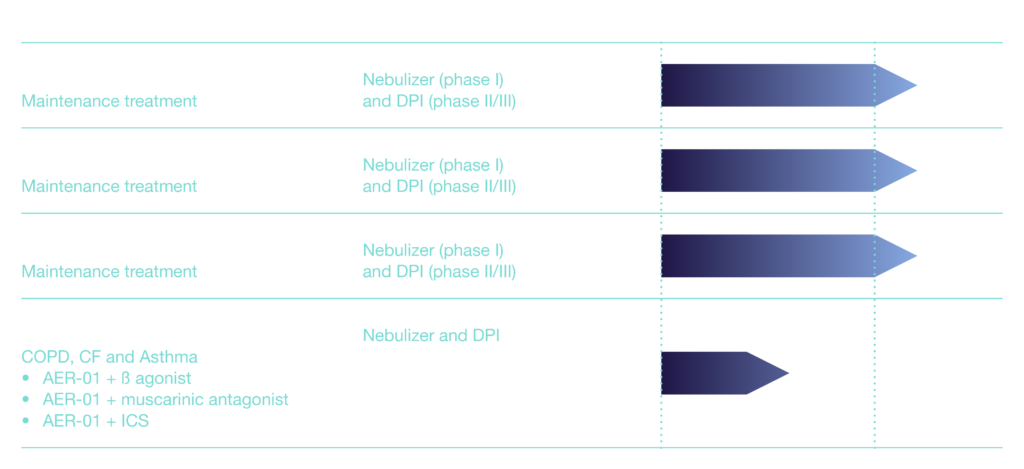
Aer Therapeutics Product Pipeline